245x Filetype PDF File size 0.48 MB Source: www.cell.com
Molecular Plant
Editor’s Highlights
Next-Generation Crop Breeding Methods
Different from conventional labor-intensive and time-consuming restricting direct utilization of genome editing tools for crop
breeding approaches such as genetic cross and mutation breeding.
screening, new breeding technologies such as double haploid
(DH) and CRISPR/Cas-mediated genome editing can greatly Twostudies recently reported innovative, rapid breeding strate-
enhance breeding efficiency and accelerate crop improvement. gies combining HI and CRISPR/Cas9-mediated genome editing
DH technology relies on an inducer line for haploid induction by a single cross, which would accelerate breeding in a wide
(HI) when pollination onto elite varieties or inbred lines. DH lines range of crops (Kelliher et al., 2019; Wang et al., 2019a). The
with desired traits are then obtained from haploids in a subse- method reported by a Syngenta research group was termed
quent generation by natural or artificial chromosome doubling HI-Edit (Kelliher et al., 2019). In their study, they first used
using mitotic inhibitors. Natural HI systems have been widely CRISPR/Cas9 to edit MATL in a maize inbred line NP2222.
usedfor breeding in maize, wheat, and barley, while engineering They found that pollens of created matl plants expressing Cas9
of HI systems is also feasible by manipulating CENTROMERIC and guide RNA (gRNA) targeting MATL could further edit
HISTONE3 (CENH3) in dicots or mutating MATL/PLA1/NLD in maternal MATL after fertilization with another commercial
rice and maize (Ravi and Chan, 2010; Kelliher et al., 2017; Liu inbred to produce haploids with new matl mutations but without
et al., 2017; Dong et al., 2018). The CRISPR/Cas9 system and the male parent genome, suggesting that matl-mediated HI, like
its variants can directly modify trait-associated genes in a short CENH3-induced HI, caused post-fertilization male genome
period and have become powerful tools for breeding in many elimination to form haploids. They then applied the HI-Edit strat-
crops nowadays. However, many elite crop varieties and inbred egy to edit four maize yield-associated genes (VLHP1, VLHP2,
lines are recalcitrant to genetic transformation and regeneration, ZmGW2-1, and ZmGW2-2). Individual Cas9-gRNA constructs
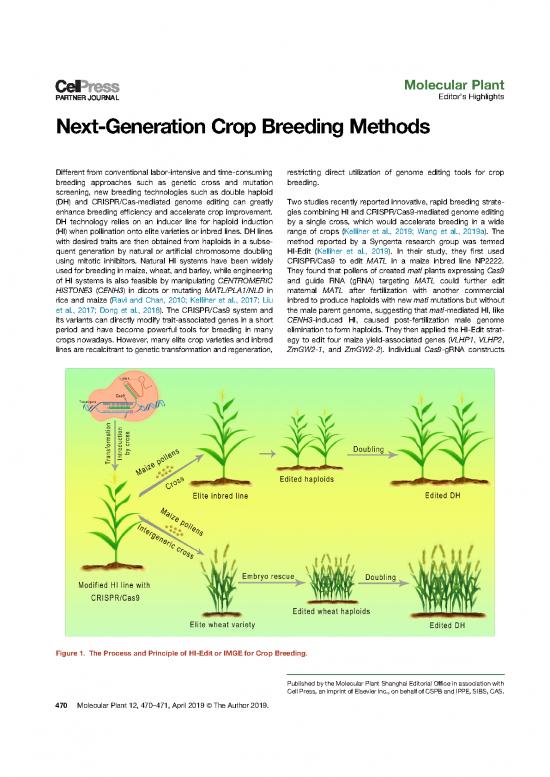
Figure 1. The Process and Principle of HI-Edit or IMGE for Crop Breeding.
Published by the Molecular Plant Shanghai Editorial Office in association with
Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, SIBS, CAS.
470 Molecular Plant 12, 470–471, April 2019 ª The Author 2019.
Editor’s Highlights Molecular Plant
were transformed into NP222, and Cas9-positive plants were reduce unwanted editing during vegetative development using
crossed with native matl HI line. F2 individuals carrying both gamete-specific promoters and improve the efficiency of both
matl mutation and Cas9 transgene were identified and pollinated genomeediting and HI (including induced by intergeneric cross)
onto the ears of several field-grown inbred lines (Figure 1). in these approaches may be the focus of future research. With
Analyses of the editing efficiency for each gene showed that the development of these highly promising approaches and
HI-Edit produced over 3% edited haploids in total from five further improvements, we trust a new era of crop breeding is
of six inbred lines. In an independent study, Wang et al. (2019a) coming of age.
reported a similar approach combining genome editing
with matl-mediated HI for maize breeding, called Haploid- Juanying Ye and Xiaofeng Cui*
Inducer Mediated Genome Editing (IMGE). They tested three
development-related genes and obtained Cas9/matl-free edited Molecular Plant
haploids for two genes in B73 background, with a higher effi- *Correspondence: Xiaofeng Cui (xiaofeng@sibs.ac.cn)
https://doi.org/10.1016/j.molp.2019.03.007
ciency (4.1%) for LIGULELESS1 (LG1), disruption of which
greatly reduced leaf angle due to the lack of leaf auricle and REFERENCES
ligules. Notably, they obtained DH seeds from three lg1 haploids Dong, L., Li, L., Liu, C., Liu, C., Geng, S., Li, X., Huang, C., Mao, L.,
resulted from natural chromosome doubling, demonstrating the Chen, S., and Xie, C. (2018). Genome editing and double-
feasibility of HI-Edit/IMGE for maize breeding. fluorescence proteins enable robust maternal haploid induction and
identification in maize. Mol. Plant 11:1214–1217.
SincetherearenoclearorthologsofMATLindicots,Kelliheretal. Kelliher, T., Starr, D., Richbourg, L., Chintamanani, S., Delzer, B.,
(2019) established an HI-Edit approach in Arabidopsis using the Nuccio, M.L., Green, J., Chen, Z., McCuiston, J., Wang, W., et al.
CENH3 HI system. They created a transgenic Col-0 line with (2017). MATRILINEAL, a sperm-specific phospholipase, triggers
native AtCENH3 replaced by ZmCENH3 transgene. After this maize haploid induction. Nature 542:105–109.
line was further transformed with a Cas9-gRNA construct target- Kelliher,T.,Starr,D.,Su,X.,Tang,G.,Chen,Z.,Carter,J.,Wittich,P.E.,
ing GLABROUS1 (GL1) that controls trichome development, Dong,S.,Green,J.,Burch,E.,etal.(2019).One-stepgenomeediting
they pollinated wild-type Ler pollens onto flowers of created gl1 of elite crop germplasm during haploid induction. Nat. Biotechnol.
mutant for HI-Editing. Analyses of haploid progenies showed 37:287–292.
high maternal HI-Edit efficiency (16.9%) and faithful inheritance Khanday, I., Skinner, D., Yang, B., Mercier, R., and Sundaresan, V.
ofeditedlociinArabidopsis,providingapotentialHI-Editstrategy (2019). A male-expressed rice embryogenic trigger redirected for
for dicot crops. In addition, they demonstrated the success of asexual propagation through seeds. Nature 565:91–95.
HI-Edit in wheat by employing a natural HI phenomenon, i.e., Liu,C.,Li,X.,Meng,D.,Zhong,Y.,Chen,C.,Dong,X.,Xu,X.,Chen,B.,
intergeneric cross of wheat with maize pollens could induce Li, W., Li, L., et al. (2017). A 4-bp insertion at ZmPLA1 encoding a
wheat haploid, and found that Cas9 expression in maize driven putative phospholipase a generates haploid induction in maize. Mol.
by pollen-specific promoters may enhance HI-Edit efficiency Plant 10:520–522.
in wheat. Ravi, M., and Chan, S.W. (2010). Haploid plants produced by
centromere-mediated genome elimination. Nature 464:615–618.
The HI-Edit/IMGE methods highlighted here might enable wide- Wang, B., Zhu, L., Zhao, B., Zhao, Y., Zheng, Z., Li, Y., Sun, J., and
spread applications of genome editing technologies in many Wang, H. (2019a). Development of a haploid-inducer mediated
commercial crop varieties. Together with two other recently re- genome editing (IMGE) system for accelerating maize breeding. Mol.
ported approaches that combine CRISPR/Cas9-created MiMe- Plant 12. https://doi.org/10.1016/j.molp.2019.03.006.
mediated apomeiosis with either ectopic expression of BBM1 Wang,C.,Liu,Q.,Shen,Y.,Hua,Y.,Wang,J.,Lin,J.,Wu,M.,Sun,T.,
or knockout of MATL for clonal propagation of hybrids Cheng,Z.,Mercier,R.,etal.(2019b).Clonalseedsfromhybridriceby
(Khanday et al., 2019; Wang et al., 2019b), these likely simultaneous genome engineering of meiosis and fertilization genes.
constitute part of next-generation crop breeding methods. To Nat. Biotechnol. 37:283–286.
Molecular Plant 12, 470–471, April 2019 ª The Author 2019. 471
no reviews yet
Please Login to review.