284x Filetype PPT File size 0.20 MB Source: atlas.physics.arizona.edu
Schrodinger Equation
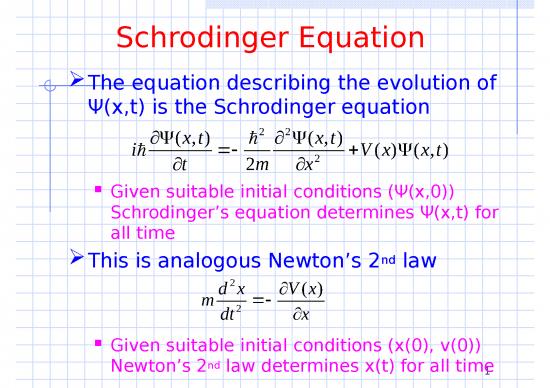
We take Schrodinger’s equation as
one of the postulates of quantum
mechanics
Schrodinger himself just “figured it
out”
Thus there is no formal proof
We rely on comparison of its
predictions with experiment to validate
it
But we’ll briefly try to motivate it
2
Schrodinger Equation
We’d like the quantum wave equation
To be consistent with de Broglie-Einstein
relations
To be consistent with E = T+V =
2
p /2m+V
To be linear in Ψ(x,t)
This means if Ψ and Ψ are solutions, then
1 2
c Ψ + c Ψ is a solution
1 1 2 2
To have traveling wave solutions for a
free particle (the case where V(x,t)=0)
3
Schrodinger Equation
The first two assumptions can be
combined into2
Ep V
2m
2k2
V
2m
The third assumption means that the
wave equation can only contain terms
like Ψ or its derivatives (no constants or
higher order powers)
4
Schrodinger Equation
Recall some of our solutions to the
classical wave equation
sin(kx t) or eikxt
Note that 2
gives a factor of k2
x2
gives a factor of
t
Thus we might guess a wave equation
that looks like 2
V
t x2
5
Schrodinger Equation
We could evaluate the constants α and β
using the exponential free particle
solution and find
(x,t) 2 2(x,t)
i t 2m x2 V(x)(x,t)
But we normally take Schrodinger’s
equation as one of the postulates of
quantum mechanics
6
no reviews yet
Please Login to review.