301x Filetype PPT File size 0.49 MB Source: www.animatedscience.co.uk
Glass Transition Temperature, Tg
4 6 2
At T , ~ 10 -10 Ns/m
g
Below T , atomic rearrange-
g
ments are frozen in.
Rigid fluid
“Moon rocks” were
produced millions of
years ago T -melting point
M
2
3.091 © H.L. Tuller-2003
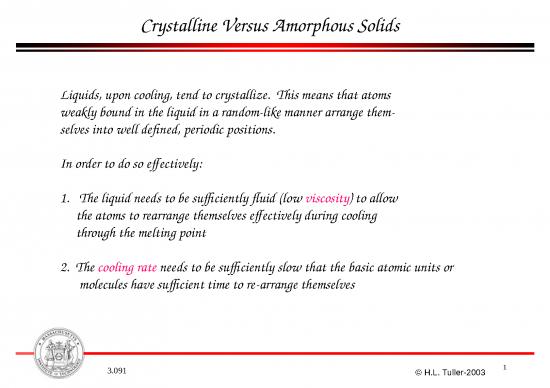
Crystalline Versus Amorphous Solids
Solids with simple structures and non-directional
bonds, e.g. metals and alkali halides, have very
low viscosity fluids above the melting point and
easily crystallize upon cooling.
Solids with complex structures and strong, highly directional bonds,
e.g. silicates, polymers, have high viscosity fluids and tend to form
amorphous or glassy solids
3
3.091 © H.L. Tuller-2003
A Crystalline Silicate
Si
O
4
3.091 © H.L. Tuller-2003
Crystalline Versus Amorphous Silicates
Silicate melts tend to be highly viscous
Variable bond
angle & length
Ordered SiO4 tetrahedra Disordered SiO4 tetrahedra
5
3.091 © H.L. Tuller-2003
Viscosity
Measure of resistance to flow:
elongation or strain, = ΔL/L
= d/dt
Liquid flow requires breaking and reformation of bonds
6
3.091 © H.L. Tuller-2003
no reviews yet
Please Login to review.