174x Filetype PDF File size 0.18 MB Source: www.briarcliffschools.org
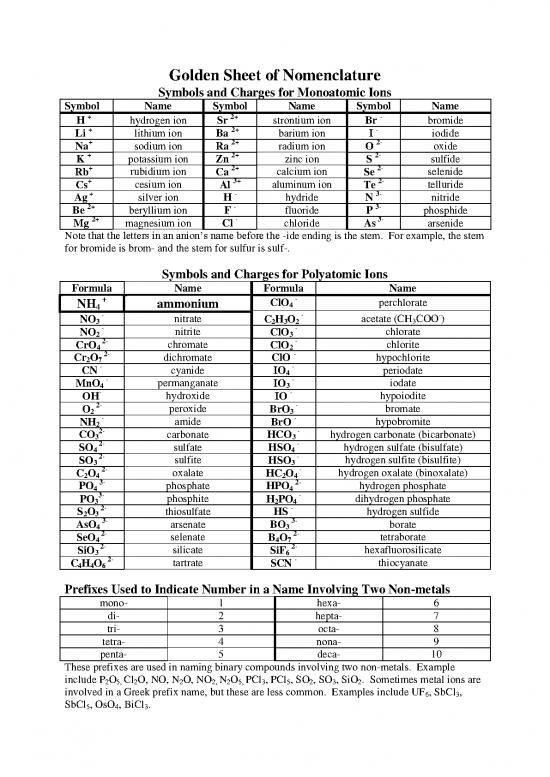
Golden Sheet of Nomenclature
Symbols and Charges for Monoatomic Ions
Symbol Name Symbol Name Symbol Name
+ hydrogen ion 2+ strontium ion - bromide
H Sr Br
+ lithium ion 2+ barium ion - iodide
Li Ba I
+ sodium ion 2+ radium ion 2- oxide
Na Ra O
+ potassium ion 2+ zinc ion 2- sulfide
K Zn S
+ rubidium ion 2+ calcium ion 2- selenide
Rb Ca Se
+ cesium ion 3+ aluminum ion 2- telluride
Cs Al Te
+ silver ion - hydride 3- nitride
Ag H N
2+ beryllium ion - fluoride 3- phosphide
Be F P
2+ magnesium ion - chloride 3- arsenide
Mg Cl As
Note that the letters in an anion’s name before the -ide ending is the stem. For example, the stem
for bromide is brom- and the stem for sulfur is sulf-.
Symbols and Charges for Polyatomic Ions
Formula Name Formula Name
+ - perchlorate
NH4 ammonium ClO4
- - -
NO nitrate CHO acetate (CH3COO)
3 2 3 2
- nitrite - chlorate
NO2 ClO3
2- chromate - chlorite
CrO4 ClO2
2- dichromate - hypochlorite
Cr O ClO
2 7
- cyanide - periodate
CN IO4
- permanganate - iodate
MnO4 IO3
- hydroxide - hypoiodite
OH IO
2- peroxide - bromate
O2 BrO3
- amide - hypobromite
NH2 BrO
2- carbonate - hydrogen carbonate (bicarbonate)
CO3 HCO3
2- sulfate - hydrogen sulfate (bisulfate)
SO HSO
4 2- sulfite 4 - hydrogen sulfite (bisulfite)
SO HSO
3 2- oxalate 3 - hydrogen oxalate (binoxalate)
CO HCO
2 4 2 4
3- phosphate 2- hydrogen phosphate
PO4 HPO4
3- phosphite - dihydrogen phosphate
PO3 H2PO4
2- thiosulfate - hydrogen sulfide
S O HS
2 3 3- arsenate 3- borate
AsO BO
4 3
2- selenate 2- tetraborate
SeO B O
4 4 7
2- silicate 2- hexafluorosilicate
SiO SiF
3 2- tartrate 6 - thiocyanate
CHO SCN
4 4 6
Prefixes Used to Indicate Number in a Name Involving Two Non-metals
mono- 1 hexa- 6
di- 2 hepta- 7
tri- 3 octa- 8
tetra- 4 nona- 9
penta- 5 deca- 10
These prefixes are used in naming binary compounds involving two non-metals. Example
include P O Cl O, NO, N O, NO N O PCl , PCl , SO , SO , SiO . Sometimes metal ions are
2 5, 2 2 2, 2 5, 3 5 2 3 2
involved in a Greek prefix name, but these are less common. Examples include UF6, SbCl3,
SbCl , OsO , BiCl .
5 4 3
Golden Sheet of Nomenclature
There is a preferred order of the nonmetals when writing them in a formula. It is:
Rn, Xe, Kr, B, Si, C, Sb, As, P, N, H, Te, Se, S, I, Br, Cl, O, F.
CO is carbon monoxide, NOT carbon monooxide. As O is tetrarsenic hexoxide, NOT
4 6
tetraarsenic hexaoxide.
Metals with more than one oxidation number
Symbol Systematic name Classical Symbol Systematic Name Classical
(stock system) Name (stock system) Name
1+ copper (I) cuprous 2+ mercury (I) mercurous
Cu Hg
2+ copper (II) cupric 22+ mercury (II) mercuric
Cu Hg
2+ iron (II) ferrous 2+ lead (II) plumbous
Fe Pb
3+ iron (III) ferric 4+ lead (IV) plumbic
Fe Pb
2+ tin (II) stannous 2+ cobalt (II) cobaltous
Sn Co
4+ tin (IV) stannic 3+ cobalt (III) cobaltic
Sn Co
2+ chromium (II) chromous + gold (I) aurous
Cr Au
3+ chromium (III) chromic 3+ gold (III) auric
Cr Au
2+ manganese (II) manganous 2+ nickel (II) nickelous
Mn Ni
3+ manganese (III) manganic 3+ nickel (III) nickelic
Mn Ni
Acid Names
Non-Oxygen Containing Oxygen Containing (Oxyacids)
Formula Name when dissolved Name as a pure Formula Name
in H O compound
2
HF hydrofluoric acid hydrogen HNO nitric acid
fluoride 3
HCl hydrochloric acid hydrogen HNO nitrous acid
chloride 2
HBr hydrobromic acid hydrogen HSO sulfuric acid
bromide 2 4
HI hydroiodic acid hydrogen HSO sulfurous acid
iodide 2 3
HCN hydrocyanic acid hydrogen HPO phosphoric acid
cyanide 3 4
HS hydrosulfuric acid hydrogen sulfide HCHO acetic acid
2 2 3 2
(CH3COOH)
Add the word acid to each name when saying or writing.
Note that it is hydrogen sulfide, NOT hydrogen sulfuride.
Diatomic Elements
The following elements are diatomic elements: Br, I, N, Cl, H, O, and F. For example,
hydrogen would be written as H2 and oxygen would be written as O2 when they are not
combined with other elements. To remember this, remember the name “BrINClHOF” (said
brinkle-hoff) or the phrase “I Have No Bright Or Clever Friends.” Lastly, you can look for the
hockey puck (Hydrogen) and stick (Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, Iodine).